Introduction
Proteins are complex, naturally occurring polymers of amino acids held together by peptide bonds. They are crosslinked between chains by sulfhydryl bonds, hydrogen bonds and van der wall forces. Proteins have highly complex chemical composition than any other biologically active compound. Proteins are made up of 20 alpha amino acids. Proteins comes in many different shapes and sizes which is important for its functioning. For example, the hemoglobin protein which carries oxygen are globular shape and the collagen protein which found in skin is fibrous shape. In this article we are going to discuss classification of proteins, structure, chemical nature and biological importance of proteins.
Classification of proteins
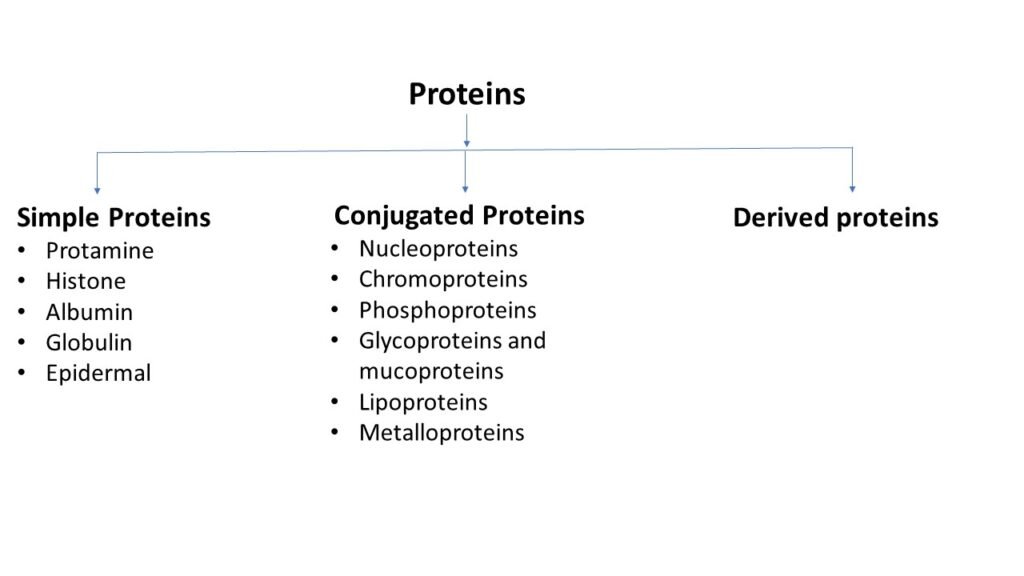
Classification based on chemical nature proteins
Simple proteins
Simple proteins on hydrolysis gives only amino acids and sometimes small carbohydrate compounds. They are further classified as follows,
Protamines: Protamine is low molecular weight protein (3000 to 10000) that contain high proportion of arginine and lysine amino acids. They do not coagulate upon heating.
Histones: These are basic proteins which have high molecular weight than protamine. They have high content of arginine, lysine and histidine. They are heat coagulable proteins. Histone provides structural support for chromosome.
Albumin: Albumin is a globular protein. It is the main protein of human blood plasma. Albumin is synthesized by liver. Albumin present in blood stream protect leaking of fluid from blood vessels to other tissues.
Globulin: Globulins are globular proteins but have high molecular weight than albumins. Globulin are insoluble in pure water but dissolve in salt solution. They play an important role in liver function and fighting infection.
Epidermal proteins: These proteins are found in skin, hair and nails. They have high content of cystine.
Conjugated proteins
These proteins contain non protein component along with amino acids hence also known as hetero proteins. On hydrolysis these proteins give amino acids and non-protein components. These components called as prosthetic groups. These prosthetic groups give some characteristic properties to proteins. Prosthetic groups may be metal ions, pigments, lipids, nucleic acids, phosphate groups and flavins. They are further classified as,
Nucleoproteins: These are the basic proteins formed by combination of histone or protamine with nucleic acid (RNA or DNA). They are present in both nucleus and cytoplasm.
Chromoproteins: These proteins are formed by colored (chromophoric) prosthetic groups. For example, haem (hemoglobin).
Phosphoproteins: These proteins with phosphate group attach to serine and threonine residuals. Example, caseins (milk) and ovovitellin (egg white).
Glycoproteins and mucoproteins: They have one or more carbohydrate units attached to the polypeptide. Glycoproteins (less than 4%) contain smaller amount of carbohydrates compare to mucoproteins (more than 4%).
Lipoproteins: The proteins forming complexes with lipids are called lipoproteins. They are found in biological membrane, plasma and brain.
Metalloproteins: These proteins are attached to the various metals. They are further classified into three groups as, metals strongly bound by proteins, metals weakly bound by proteins and metals which do not bind by proteins.
Derived proteins
These are the proteins derived from simple or conjugated proteins by physical or chemical actions. Derived proteins classified in two types one is primary derived proteins and second is secondary derived proteins. Primary derived proteins do not show any major difference in size from their parent molecules. For example, myosan derived from myosin. Secondary derived molecules have smaller size than parent molecules. For example, globulose derived from globulin.
Classification based on shape of proteins
- Fibrous proteins
- Globular proteins
Fibrous proteins are ribbon or fibrous in shape. Most fibrous proteins play structural or protective roles. For example, collagen. Globular proteins are spherical or ovoid shape. They mostly play an important role in transportation. For example, hemoglobin.
Structure of proteins
Proteins are made up of amino acids. These amino acids go through various stages of folding to form their shape and structure. The protein structure can be categorized in four categories depending on their complexity.
Primary protein structure is the linear sequence of amino acids which are bound together by covalent peptide bonds. These bonds are formed between the N terminal (amino terminal) and C terminal (carboxyl terminal).
Secondary protein structure is the arrangement of polypeptide chains by hydrogen bonds between the hydroxyl group and the hydrogen molecules. There are two types of these arrangements, one is alpha helical structure and second beta pleated structure.
In alpha helical structure a spiral shape is formed by hydrogen bond between carbonyl group (=C=O) and amino group (=N-H). The polypeptide of alpha helix coil around the long axis. The hydrogen bonds are parallel to the axis and the side chains are projected outside. Each turn of alpha helix contains 3.6 amino acid. Each amino acid is spaced 0.15 nm from each other.
The beta pleated sheets are formed by hydrogen bond between carboxyl group of one amino acid on one sheet and hydrogen molecule of amino acid on another sheet. In beta pleated structure the polypeptide chains are bound together by hydrogen bonds in such a way that they form a sheet like structure.
Tertiary structure of proteins is the 3-dimensional structure of folding chain of polypeptides. This folding is occurring due to the interaction of R groups (side chain) of amino acids. The interactions are due to the hydrogen bonds, hydrophobic bonds and ionic bonds.
Quaternary structure of proteins contains two or more polypeptides which are linked together by non-covalent bond to form a functioning unit. At this stage protein is fully functional to perform its specific roles. For example, hemoglobin, insulin.
Chemical nature of proteins
Hydrolysis
On hydrolysis protein gives free amino acids. Hydrolysis can be done by acids like HCl, H2SO4 or bases like NaOH, KOH. Ninhydrin and Biuret tests are used to determine the extent of hydrolysis. Ninhydrin test is negative for protein but on hydrolysis it is positive as free amino acids are formed.
Biuret test
This test is used for the identification of proteins. When proteins treated with Biuret reagent it gives a violet color. Biuret reagent is made up of copper sulphate in an alkaline medium.
Xanthoproteic test
On treatment with concentrated nitric acid proteins give yellow precipitate which further turns to orange color when treated with alkali. The aromatic amino acids of proteins give yellow color on nitration.
Millions test
The phenolic group of tyrosine of proteins reacts with mercuric sulphate in the presence of sodium nitrate (NaNO3) and sulphuric acid (H2SO4) gives red color.
Heat test
When protein solution is heated in boiling water, it gets coagulated and losses its biological activity. For example, boiling of eggs.
Precipitation test
Proteins can be precipitated by using following reagents.
- Salts: Ammonium sulphate and sodium chloride
- Organic solvents: Acetone, alcohol
- Heavy metal ions: Sodium tungstate, copper or mercury salts
- Acids: Trichloroacetic acid, acetic acid, hydrochloric acid
Denaturation of proteins
Denaturation is the disruption of secondary, tertiary and quaternary structure of protein which results in change of physical, chemical and biological characteristics of proteins. For example, when eggs are boiled protein gets coagulated.
Biological importance of proteins
Proteins are essential for various vital biological processes. Based on their biological role they are categorized as follows,
Enzymes: The proteins which catalyze biochemical reactions called enzymes or catalytic proteins. Example, amylase, urease, catalase, alcohol dehydrogenase.
Transport proteins: The proteins which are responsible for transportation of various essential nutrients or gases to various parts of the body called transport proteins. Example, hemoglobin transports oxygen, ceruloplasmin transports copper in blood.
Regulatory proteins: The proteins which control metabolic pathway are called as regulatory proteins. For example, insulin which regulates sugar metabolism.
Defense proteins: The proteins which are responsible to protect the organism against invasion by other species or toxic substances are called as antibodies or defense proteins. For example, immunoglobulins fight bacterial or viral infections. Fibrinogen and thrombin help in blood clotting to prevent blood loss due to injury.
Structural proteins: The proteins which maintain the native form of cells or tissue and provide strength to them are called structural proteins. For example, collagen, keratin and elastin.
Muscle proteins: The proteins which are required for mechanical work are called as muscle proteins. These proteins have ability to contract, to change shape and to move. For example, actin and myosin- act as a contractile protein in skeletal muscle system.
Storage proteins: The proteins which are required for storage of nutrients are called as storage proteins. For example, casein (milk), ovalbumin (egg) and ferritin (bacteria and plants).
Conclusion
Proteins play an important role in the structure and function of living organisms. It includes catalyzing biochemical reactions, providing structural support, protection from infection, store nutrients, regulating metabolic pathways, etc. Proteins are essential for life. Understanding the various functions of proteins is crucial for maintenance and improvement of overall health and wellbeing.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us

1 thought on “Proteins: Classification, Structure, Chemical Nature and Biological Importance of Proteins”