Introduction
Lipids are fatty organic compounds which make up the building blocks of structure and function of cells of living organisms. Lipids are insoluble in water and soluble in nonpolar solvents. Like other biomolecules lipids also plays an important role in human life and other living organisms. Lipids serve various functions in biological systems, including energy storage and structural components of cell membrane. The structural diversity of lipids allows them to the form and function of cells, tissues and organisms.
In this article we will cover classification of lipids, structure and functions of lipids, role of lipids in health and disease.
Classification of lipids
Lipids can be classified in four ways,
- On the basis of chemical composition
- On the basis of fatty acids
- On the basis of requirements
- On the basis of sources
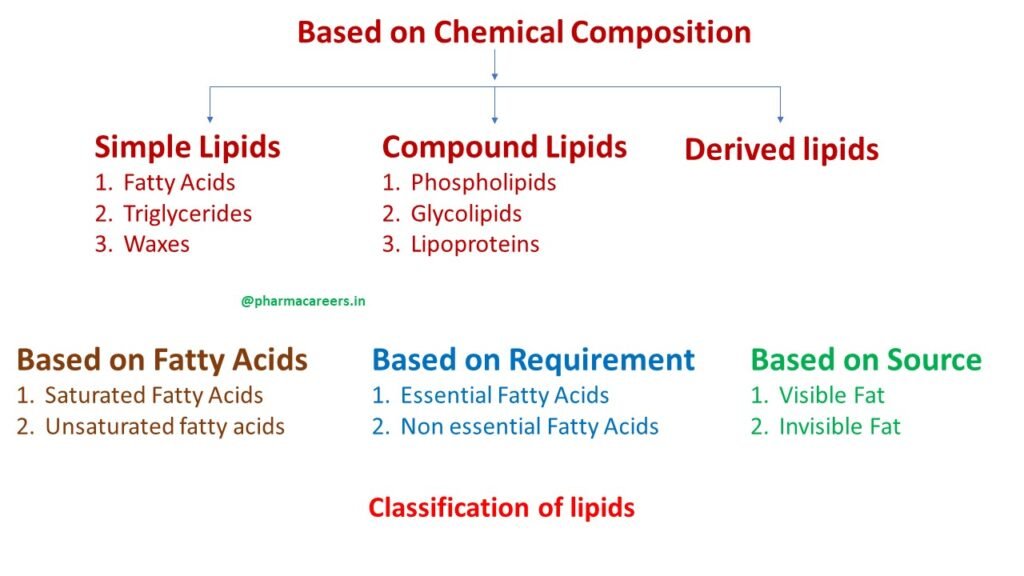
On the basis of chemical composition
Lipids on the basis of chemical composition divided into 3 main categories: simple lipids, compound lipids and derived lipids.
Simple lipids
Simple lipids are ester of fatty acids with various alcohol, it is also termed as neutral fats or triglyceride. Simple lipids make up almost 98-99% of food, body fats and oils. There are 3 sub types of simple lipids: fatty acids, triglycerides and waxes.
Fatty acids
Fatty acids are the simplest form of lipids. Fatty acids are a long chain of hydrocarbons with one carbonyl group. They are amphipathic, having both polar and nonpolar ends. The alkyl chain present in structure can be saturated or unsaturated.
Triglycerides
Triglycerides are tri-esters of fatty acids and glycerol. They are nonpolar and hydrophobic in nature. Triglycerides of two types, simple and mixed. Simple triglycerides contain only single type fatty acids and mixed type of triglycerides contain two or more types of fatty acids.
Waxes
Waxes are esters of long chain fatty acids and long chain alcohol. They are formed by the esterification of long chain fatty acids and monohydroxy alcohol of high molecular weight. Waxes are solid at room temperature and water insoluble.
Compound lipids
The compound lipids or complex lipids contain some other organic compounds in addition to fatty acids and glycerol. There are three types of compound lipids phospholipids, glycolipids and lipoproteins.
Phospholipids
Phospholipids are composed of fatty acids, glycerol or sphingosine, phosphate and alcohol attached to phosphate. They are amphipathic in nature. Phospholipids are primary components of cell membrane.
Glycolipids
Glycolipids contain carbohydrates in combination with fatty acids and glycerol. They are consisting of hydrophobic lipid tail and hydrophilic carbohydrate head.
Lipoproteins
Lipoproteins are consisting of lipids and proteins. They play an important role in transportation lipids through the blood streams. They are categorised into four types based on their densities.
- Chylomicrons
- Very Low-Density Lipoproteins (VLDL)
- Low-Density Lipoproteins (LDL)
- High-Density Lipoproteins (HDL)
Derived lipids
Derived lipids are substances liberated during hydrolysis of simple and compound lipids but they still retain the properties of lipids. They include sterols, fatty acids and alcohol.
Sterols are solid alcohols and form esters with fatty acids. Base on origin they are classified as cholesterol (in animal) and phytosterol (in plants).
On the basis of fatty acids
Lipids can be classified as saturated fatty acids and unsaturated fatty acids on the basis of fatty acids.
Saturated fatty acids
Saturated fatty acids do not have any double or triple bonds. They are simple, unbranched and linear chain of methylene groups (CH2) connected with carbon-carbon single bond and one carboxylic acid.
The general formula for saturated fatty acids is CH3-(CH2)n-COOH. Where n is number of methylene groups.
Examples lauric acid, myristic, palmitic and lignoceric acids.
Unsaturated fatty acids
Unsaturated fatty acids have one or more double or triple bonds. Based on number of double bonds they classified as monounsaturated fatty acids and polyunsaturated fatty acids.
Monounsaturated fatty acids (MUFA) contain only one double bond. Example, palmitoleic acid. Polyunsaturated fatty acids (PUFA) contain more than double bond example, linolenic acid.
Based on requirement of body
On the basis of requirement lipids are divided into two types essential and non-essential fatty acids.
Essential fatty acids
Fatty acids which cannot be produced or synthesized in human body are called as essential fatty acids. They are essential for complete nutrition hence supplied through a diet. They are polyunsaturated fatty acids (PUFA). The two important essential fatty acids are linolenic acid linoleic acid.
Non-essential fatty acids
Non-essential fatty acids are synthesized in human body. It includes palmitic acid, oleic acid and butyric acid.
On the basis of sources
On the basis of sources lipids are divided into two types visible and invisible lipids. Visible fats are the fats which we can see in our food like butter, cooking oil, etc. Invisible fats are the hidden fats which are not visible but present inside the food.
Chemical nature of lipids
Hydrolysis
Triglycerides reacting with water gives carboxylic acid and alcohol.
Saponification
Triglycerides on hydrolysis with alkali (NaOH or KOH) or lipase enzyme gives two products. One is soap or fatty acid salt of sodium or potassium and alcohol.
Hydrogenation
The breakage of double bonds of unsaturated fatty acids on reaction with hydrogen is known as hydrogenation of fatty acids. This turns unsaturated fatty acids into saturated fatty acids.
Halogenation
Free or combined fatty acids on reaction with halogen gain double bonds which causes decolourization of halogen solution.
Rancidity
When fat comes in contact with air and moisture in presence of light for a sufficient time period. The fat gets oxidized and become rancid. It liberates free fatty acids. To prevent rancidity small amount of antioxidant is added into the fat.
Qualitative test for lipids
Solubility: Lipids are soluble in organic solvents. Example chloroform, alcohol, ether.
Translucent spot on paper: When a drop of oil is applied on paper, semi-transparent spot appears on paper.
Formation of acrolein: When fat is heated in the presence of potassium bisulphate, the glycerol part of fat get dehydrated which gives acrolein.
Emulsification: When fat or oil is shaken with water the oil is finely dispersed in water and form an emulsion.
Iodine absorption test: This test is for unsaturated fatty acids. When a drop of iodine is added to fat it gets decolourized if unsaturated fatty acid is present.
Methods used to study lipids
Saponification value: Saponification value or saponification number is the number of milligrams of potassium hydroxide or sodium hydroxide required to saponify one gram of fat.
Acid value: It is the number of milligrams of alkali required to neutralize free fatty acid present in one gram of oil or fat.
Iodine number: Iodine number represents the amount of unsaturated fatty acids present in total fat or oil. It is the quantity of iodine that required to saturate fatty acids in 100gm fat or oil.
Acetyl number: It is the milligrams of potassium hydroxide required for neutralization of acetic acid formed on saponification of 1gm of acetylated fat or oil.
Reichert-Meissel number: It is the number of millilitres of 0.1N alkali required to neutralize the soluble volatile fatty acids derived from 5gm of fat or oil.
Polenske number: It is the number of millilitres of 0.1N alkali required to neutralize the insoluble volatile fatty acids derived from 5gm of fat or oil.
Unsaponifiable matter: Unsaponifiable matter is the part of fat or oil which does not get saponified by alkali.
Biological role of lipids
The lipids are rich source of energy when stored in adipose tissue. It is one of the major biomolecules which plays various important roles in human body.
Chemical messengers
Chemical messengers pass the information form organelles to other cells. They further attach to receptors on the cell surface and bring out the change that led to action. lipids are small water insoluble molecule hence best candidate to serve as chemical messenger. Lipids in their esterified form infiltrate cell membrane transport signal to other cells.
Energy storage
Lipids are stored in the form of triacyclglycerols or triglycerides which is inert substance and made up of fatty acids and glycerol. During fasting triglycerides release fatty acids to provide energy and to form structural component for cells. One gram of lipid on complete breakdown releases about 9 kcal of energy.
Structural component of cell membrane
The plasma membrane of cell is made up of lipid bilayer. The lipid bilayer is made up of amphipathic glycerophospholipid molecules. The glycolipids and phospholipids present in cell membrane act as structural component of cell membrane. Some non-glyceride lipids like sphingomyelin and sterols are responsible for membrane flexibility.
Temperature maintenance
The lipid layer present under the skin helps in insulation and protection from cold and heat. Body temperature is mainly maintained by brown fat.
Inflammation
Linoleic acid and linolenic acid are precursors pf prostaglandins, thromboxane, leukotriene which play important role in pain, fever and inflammation.
Fat soluble vitamins
Essential nutrients like fat soluble vitamins- A, D, E and K are stored in lipids.
Conclusion
Lipids are one of the crucial biomolecules which has diverse role in our body. They store energy, build cell structure and participate in important processes like inflammation and blood clotting. Study of lipids help us to improve health and treat health issues.
Frequently asked questions
What is saponification?
Saponification is a chemical process in which fats or oils reacts with alkaline substance normally sodium hydroxide (NaOH) or potassium hydroxide (KOH) to produce fat and glycerol.
Which is lipid soluble vitamins?
Vitamins A, D, E and K are the lipid soluble vitamins.
How lipids are formed?
Lipids are formed in the body by a process known as lipogenesis. In the lipogenesis excess carbohydrates present in the body converted into lipids.
Where lipids are produced in cell?
Lipids are produced in endoplasmic reticulum (ER).
What are the sources of lipids?
Some common sources for lipids are, red meat, poultry, fish, egg, milk & milk products.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us

1 thought on “Lipids: Classification, Chemical Nature and Biological Importance”