Introduction
Carbohydrates or carbs are the biomolecules containing sugars, starches and fibres found in some foods. Chemically carbohydrates are polyhydroxy aldehydes or ketones, or compounds derived from their hydrolysis. Carbohydrates generally contain carbon, hydrogen and oxygen. The general formula for Carbohydrates is Cn(H2O)n. Where n is number of carbon atoms. Carbohydrates are also known as saccharides; saccharides word comes from Greek term sakchron which means sugar. It provides energy to the body. Each gram of Carbohydrates provides 4 calories. In this article we will see classification, chemical nature and biological role of Carbohydrates.
Classification of Carbohydrates
Carbohydrates contain carbon, hydrogen and oxygen in their chemical structure. They are made up of two basic compounds: one is Aldehydes which are double bonded carbon and oxygen atoms plus hydrogen atoms. Second is Ketones which are double bonded carbon and oxygen atoms, with two additional carbon atoms.
Carbohydrates classified into simple and complex based on their chemical structure and degree of polymerization.
Monosaccharides, Disaccharides and Oligosaccharides are simple carbohydrates, and polysaccharides are complex carbohydrates.
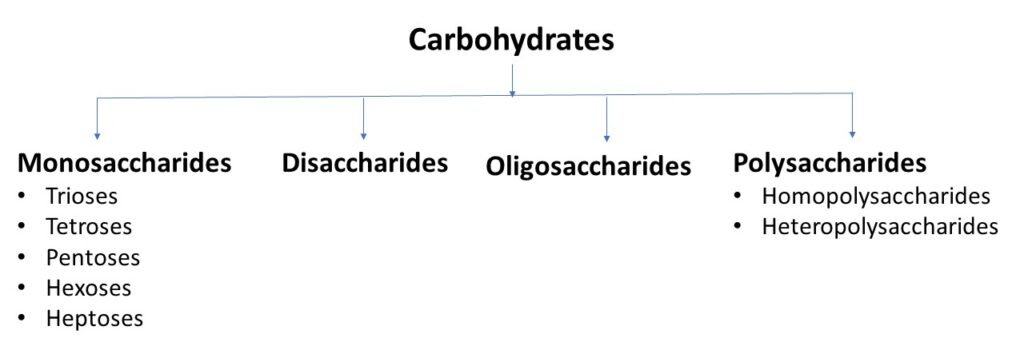
Monosaccharides
Monosaccharides (mono = one, sacchar = sugar), are the simple sugars which contain one monomer of carbohydrates. They can not be further hydrolysed to simpler compounds. Monosaccharide can be further classified on the basis of their functional group and number of carbon atoms present in their structure.
Classification based on functional group
- Aldoses: Contains aldehyde as functional group. Ex. Glucose.
- Ketoses: Contains keto as functional group. Ex. Fructose.
Classification based on number of carbon atoms
- Trioses: Contain 3 carbon atoms. Ex. Glyceraldehyde.
- Tetroses: Contain 4 carbon atoms. Ex. Erythrose.
- Pentoses: Contain 5 carbon atoms. Ex. Ribose, Xylose.
- Hexoses: Contain 6 carbon atoms. Ex. Glucose, Mannose
- Heptoses: Contain 7 carbon atoms. Ex. Glucoheptose.
Disaccharides
Carbohydrates containing two monosaccharides and giving two monomeric units on hydrolysis are called as disaccharides.
For example: maltose, lactose and sucrose
Oligosaccharides
Carbohydrates that give 3-10 monomers on hydrolysis are called as oligosaccharides.
For example: raffinose, maltotriose
Polysaccharide
Carbohydrates that give more than ten monomers on hydrolysis called as polysaccharides. Polysaccharides are made up of different types of sugars linked to each other via glycosidic linkage. These are the complex Carbohydrates of high molecular weight, low solubility in water and on hydrolysis give simple sugars.
Polysaccharides act as food source in plants and animals. Examples,
- Starch, it is mainly composed of amylose and amylopectin. Amylose is a linear chain and amylopectin is branched chain.
- Glycogen, which stores energy in liver and muscles. It is animal starch but has more extensive branching than starch.
- Cellulose, it is the main component of plant cell wall. It is a fibrous polysaccharides containing high tensile strengths.
Based on the product of hydrolysis polysaccharides are classified into two classes, as follows:
Homopolysaccharides
Polysaccharides which on hydrolysis gives only one type of monomers are called as homopolysaccharides. Example, starch, cellulose, chitin.
Heteropolysaccharides
Polysaccharides which on hydrolysis gives two or more types of monomers are called as heteropolysaccharides. Example, heparin, agar, alginic acid
Chemical nature and qualitative test of carbohydrates
Reactions of carbonyl group
Carbohydrates having free or potential carbonyl group (C=O) act as reducing agent. In case of polysaccharides the potential carbonyl group is blocked in the formation of glycosidic bonds, hence they are nonreducing.
Reducing sugars reduce certain metal ions like copper, mercury, etc. Based on this property there are some qualitative tests for carbohydrates, such as Benedict’s test, Barfoed’s test, Fehling’s test.
Benedict’s test
When carbohydrates are heated with alkaline copper sulphate (CuSO4), copper ions get reduced and red precipitate of cuprous oxide (Cu2O) is formed.
CuSO4 = Cu2O + 2H2O + [O]
All reducing sugars gives this test positive.
Barfoed’s test
When reducing monosaccharide reacts with copper ion in presence of weak acidic condition it reduces copper ion and gives brick red precipitate.
Fehling’s test
Reducing sugars in the presence of Fehling’s solution reduces copper ions and gives red precipitate. Fehling’s solution is the mixture of copper sulphate, potassium hydroxide (KOH) and sodium potassium tartrate (Rochelle salt) in aqueous medium.
Dehydration
Carbohydrates in the presence of concentrated sulphuric acid (H2SO4) gets dehydrated and gives furfural or its derivatives. Based on this principle Molisch test is widely used to identify Carbohydrates.
Molisch test
This is general test for identification of all types of Carbohydrates. In this test carbohydrates are hydrolysed into furfural and its derivatives in the presence of concentrated sulphuric acid (H2SO4). The product formed reacts with alpha naphthol to give purple complex.
Reduction
The carbohydrates can be reduced to alcohol by reagents such as hydrogen and platinum. Such reduced derivatives of carbohydrates are called as alditols. For example, sorbitol, glycerol and rabitol.
Oxidation
Carbohydrates on oxidation produces acid. The oxidation product depends upon oxidising agents used in reaction. For example,
- Glucose in the presence of bromine gives gluconic acid
- Glucose in the presence of platinum and oxygen give glucuronic acid
- Glucose in the presence of nitric acid gives glucosaccharic acid

Mucic acid test
Galactose or lactose on oxidation gives galactosaccharic acid (mucic acid), in the presence of concentrated nitric acid. The mucic acid formed is insoluble and crystalline in nature. Crystals are colourless and broken glass type.
Iodine test
It is a rapid test for identification of polysaccharides. Starch gives blue colour with iodine and glycogen gives reddish brown complex with iodine.
Optical isomerism
Carbohydrates contain asymmetric carbon atom, hence they are optically active. A carbon atom is said to be asymmetric when its mirror images are non-superimposable.
These compounds have a tendency to rotate the plane of polarized light either clockwise (dextro rotatory) or anticlockwise (leavo rotatory). This pair of compounds rotating plane of polarised light in opposite direction is known as enantiomers.
The extent of optical rotation is measured by instrument named polarimeter. The polarimeter specifically designed for examination of sugar solution is known as saccharimeter. For any compound specific rotation is constant and it is used in the qualitative analysis of that compound.
| Sugars | Specific rotation |
| D- Glucose | +52.50 |
| D- Fructose | -92.30 |
| D- Galactose | +80.20 |
| D- Mannose | +104.20 |
| D- Xylose | +18.80 |
| Sucrose | +66.50 |
| Lactose | +52.50 |
| Maltose | +137.00 |
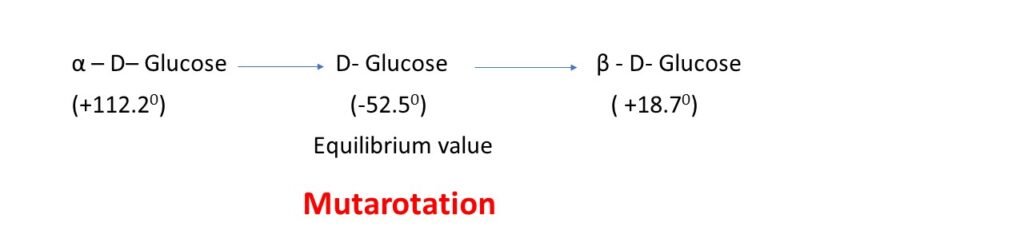
Mutarotation: When isomers of the same carbohydrates are dissolved in water, their optical rotation changes gradually with time and finally achieves equilibrium value. This phenomenon is called mutarotation.
Biological importance of carbohydrates
- Carbohydrates are the main source of energy for humans and constitute about 45-60% of their meal.
- Carbohydrates serves as storage molecules. For example, glycogen in animals and starch in plants.
- Carbohydrates are main constituents of cell structure like in protein- glycoproteins, in lipids- glycolipids.
- Carbohydrates are basic material for organic compounds like amino acids, nucleic acids and lipids.
- Cell walls of plant and bacteria are made up of cellulose (carbohydrate).
Conclusion
Carbohydrates are the important biomolecules in human life and play an important role in survival. Carbohydrates not only provides energy but also, they are the main components for preparation of various macromolecules necessary for human life, such as proteins, nucleic acids and lipids. Some naturally occurring carbohydrates like heparin, hyaluronic acid are very useful biochemically.
Frequently asked questions
What are the four types of carbohydrates?
Carbohydrates are mainly divided into four types as, monosaccharides, disaccharides, oligosaccharides and polysaccharides.
What is the structure of carbohydrates?
Carbohydrates are made up of carbon, hydrogen and oxygen. All carbohydrates include aldehyde or ketone group and hydroxyl group. The general formula for Carbohydrates is Cn(H2O)n. Where n is number of carbon atoms.
What is the smallest unit of carbohydrates?
Monosaccharides are the smallest unit of carbohydrates.
What are the main functions of carbohydrates?
The primary functions of carbohydrates are energy production, energy storage, building of macromolecules and lipid metabolism.
One gram of carbohydrates provides how much energy?
One gram of Carbohydrates provides 4 calories.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us

1 thought on “Carbohydrates: Introduction, Classification, Chemical Nature and Biological Importance”