Introduction
The structural isomerism arises when each atom of a molecule can be arranged in different order. Isomerism is the phenomenon where two or more compounds have same molecular formula but different physical and chemical properties. Isomers are the compounds which have different physical and chemical properties but same molecular formula. Isomerism in organic compounds can be classified in two broad categories, structural isomerism and stereoisomerism. In this article we will study structural isomerism in organic compound.
Structural isomerism
The structural isomerism is also called as constitutional isomerism, it arises when atoms within a molecule are arranged in different orders. The structural isomers are a compound having same molecular formula but different structural formulas. The structural isomerism in organic compound is further divided into,
- Chain isomerism
- Positional isomerism
- Functional isomerism
- Metamerism
- Tautomerism
Chain isomerism
Chain isomerism occurs due to different arrangements of carbon atoms which may lead linear or branched chains. The chain isomers have same molecular formula but different structure (linear branch). Chain isomers have same chemical properties but different chemical properties. for example, branched chain isomers have lower boiling point compare to linear chain. Linear chain isomers have more surface area of contact hence the intramolecular forces of attraction are maximum.
Example, there are two isomers of butane, C4H10. In one of them carbon atoms lie in straight chain and in another one carbon atoms lie in branched chain.
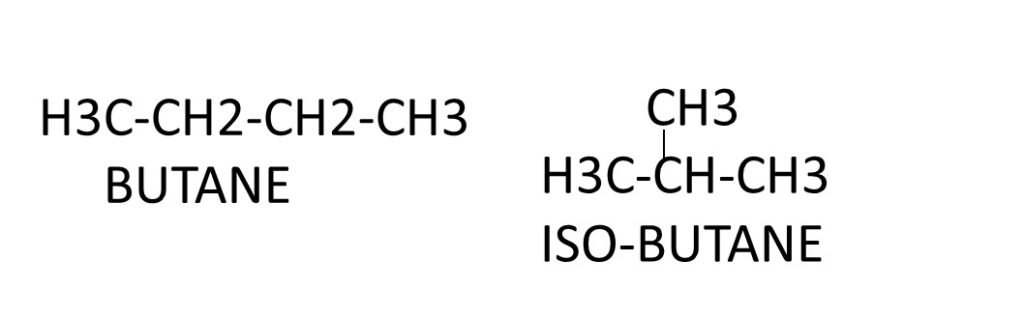
Number of chain isomers possible for alkane.
| Molecular formula | Possible number of chain isomers |
| Butane (C4H10) | 2 |
| Pentane (C5H12) | 3 |
| Hexane (C6H14) | 5 |
| Heptane (C7H16) | 9 |
| Octane (C8H18) | 18 |
| Nonane (C9H20) | 35 |
| Decane (C10H22) | 75 |
Positional isomerism
In positional isomerism important groups move around a carbon skeleton but do not change the basic carbon structure. Positional isomerism occurs due to different positions of side chains, substituents, functional groups, double bond, triple bonds, on the parent chain.
Example,
Pentane (C5H12) shows three isomers,
- n-Pentane: A straight chain.
- Isopentane: A branched chain with a methyl group.
- Neopentane: A highly branched structure.
Due to double bond position butene (C4H8) have two isomers, one is but-1-ene and another one is but-2-ene.
Functional isomerism
In functional isomerism isomers contain different functional groups. The functional isomers have same molecular formula but have different functional groups.
Example,
Dimethyl ether (H3C-O-CH3) is the functional isomer of the ethyl alcohol (H3C-CH2-OH). Both have some molecular formula, C2H6O. But, different functional groups.
Metamerism
In metamerism different alkyl groups are attached to same functional group.
Example,
Alkyl group attached to the ether functional group,
Diethyl ether (H3C-CH2-O-CH2-CH3) is isomer to the Methyl propyl ether (H3C-O-CH2-CH2-CH3).
Tautomerism
In tautomerism there is reversible transformation between different structural forms of a molecule, usually through the relocation of a hydrogen atom within the compound. Tautomer are the unique structures that coexist in dynamic equilibrium with each other due to rapid interconversion from one form to another. tautomerism is also known as desmotropism or kryptotropism or prototropy or allelotropism.
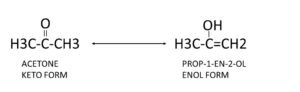
Keto-enol tautomerism: It involves two forms, a ketone structure and an enol structure. Acid or base catalysts facilitate their interconversion. The carbonyl compound containing at least one α-hydrogen atom shows keto-enol tautomerism. An alcoholic group on C=C is called as enol.
Summary
Isomers have same molecular formula but different physical and chemical properties. Study of isomerism in organic compounds shows how molecules can have different arrangement having same molecular formula. This diversity helps in the field of medicine and material science. Understanding structural isomerism is crucial for predicting chemical behaviour, reactivity, and physical properties.
For more regular updates you can visit our social media accounts,
Instagram: Follow us
Facebook: Follow us
WhatsApp: Join us
Telegram: Join us
